Silver Nitrate Reacts With Potassium Phosphate
Sulfur is the tenth most abundant element by mass in the universe. Structure of Ca 3 PO 4 2.

Silver Nitrate Potassium Phosphate Balanced Equation Brainly In
Zinc reacts with silver nitrate to produce zinc nitrate and silver.

. Water soluble calcium phosphate is an important material for plant growth and is commonly dispersed in the soil. Potassium chromate is used as an indicator. Potassium oxide reacts with water to produce potassium hydroxide.
It is abundant multivalent and nonmetallicUnder normal conditions sulfur atoms form cyclic octatomic molecules with a chemical formula S 8Elemental sulfur is a bright yellow crystalline solid at room temperature. BaCl2 2AgNO3 arrow BaNO32 2AgCl BaCl2 2AgNO3 arrow BaNO32. Calcium phosphate is insoluble in water but soluble in acids.
Various halide compounds are tested using silver nitrate solution. Sulfur or sulphur in British English is a chemical element with the symbol S and atomic number 16. Silver in brain and spinal cord sections from rats treated with.
Even with low doses and short survival periods silver accumulated in large motorneurons in. Acetic acid to form yellow precipitate of lead chromate. It is used in natural farming.
Determine the minimum concentration of KOH required for precipiration to begin in each case. Confirmation of Bromide Br- a Silver nitrate test. The reaction between an acid and a base can be represented by the general word equation shown below.
Aqueous solutions of potassium hydroxide and phosphoric acid react to produce potassium phosphate in aqueous solution and water. Acid base salt water. Silver nitrate was visualized by physical appearance.
In this method silver nitrate is used as titrant and chloride ion solution as analyte. Some include Kl KBr and KCl. At the end point when all chloride ions are consumed by silver ions reddish brown colored precipitate is formed by reaction of silver ions and chromate ions.
FeSs HCLaq FeCl2s H2Sg Solid ironII sulfide and aqueous solution of hydrochloric acid react to produce solid ironII chloride and gaseous hydrogen sulfide. 00025 M FeNO32 c. A C6H12 O6 6 O2 6 CO 2 6 H2O b Combustion 13.
B Manganese dioxide test. A student mixes two solutions one containing 3550 g of silver nitrate and the other containing 001133 moles of calcium chloride. Potassium hydroxide is used to precipitate each of the cations from their respective solution.
Chloride ion Cl-Add dilute nitric acid then add silver nitrate solution A white ppt of silver chloride formed Iodide ion I-Add dilute nitric acid then add silver nitrate solution A yellow ppt of silver iodide formed Identifying Gases Gas Colour and Odour Test Observations Hydrogen H 2 Colourless and odourless Place a lighted splint at. Sodium hydroxide potassium hydroxide calcium. The two solutions react according to.
A base is a substance that contains hydroxide 1 A base is often a compound made up of a metal and hydroxide. Observation- A deep blue color will be developed. Glucose C 6H12 O6 reacts with oxygen gas to produce carbon dioxide and water.
Its a direct titration method. Observation- White precipitate will be formed that can be dissolved in ammonium hydroxide NH 4 OH Anions present. The distribution of silver in the central nervous system was heterogeneous.
Calcium phosphate may dissolve slightly in CO2-containing water. Hydrazine N2H4 is used as rocket fuel. Then add solid potassium iodide and starch solution to the boiled mixture.
When halogen reacts with silver nitrate solution precipitation will be formed and it varies in colour depending upon the type of halides. Sodium chromate reacts with lead acetate in presence of dil. A Zn 2 AgNO 3 Zn NO 32 2 Ag b Single Replacement 12.
Anions present- Chloride Cl- Test for confirmation- Dissolve silver nitrate in the water extract. How many grams of barium nitrate can be produced when 186 grams of barium chloride reacts with 204 grams of silver nitrate. A precipitate is expected to be formed when an aqueous solution of sodium sulfate is added to an aqueous solution of A ironIII chloride B potassium chloride C magnesium.
Silver Bromide with pale yellow precipitate. Uses of Calcium phosphate Ca 3 PO 4 2. A precipitation B acid-base neutralization C oxidation-reduction D gas evolution E no reaction.
Some include Silver Fluoride with no precipitate. 0015 M CaCl2 b. The silver penetrated the blood brain barrier and accumulated in the neurons and glia.
What type of a reaction occurs when a potassium nitrate solution is mixed with a barium acetate solution. In general an acid reacts with a base to produce a salt and water. Bromides on reaction with silver nitrate solution forms a pale yellow precipitate of silver bromide which is sparingly soluble in ammonium hydroxide.

How To Write The Net Ionic Equation For K3po4 Agno3 Ag3po4 Kno3 Youtube
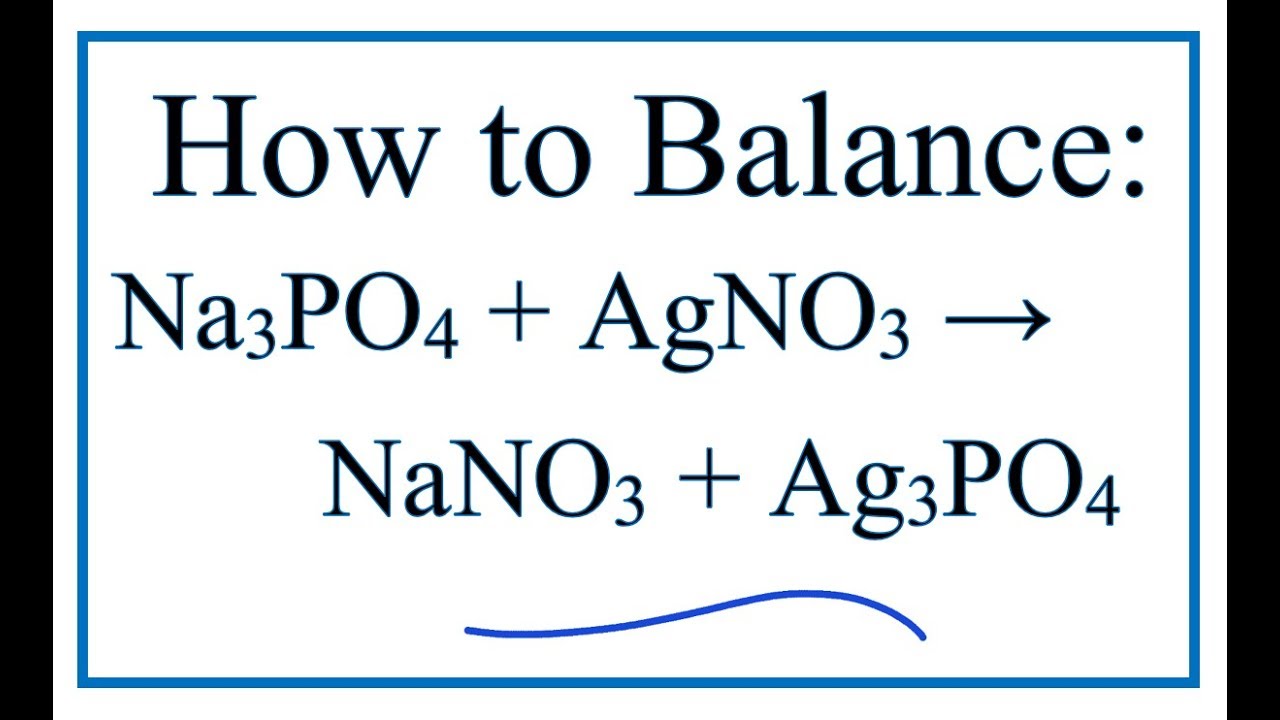
How To Balance Na3po4 Agno3 Nano3 Ag3po4 Sodium Phosphate Silver Nitrate Youtube

How To Write The Net Ionic Equation For K3po4 Agno3 Ag3po4 Kno3 Youtube

Solved Silver Nitrate And Potassium Phosphate React To Form Chegg Com
Comments
Post a Comment